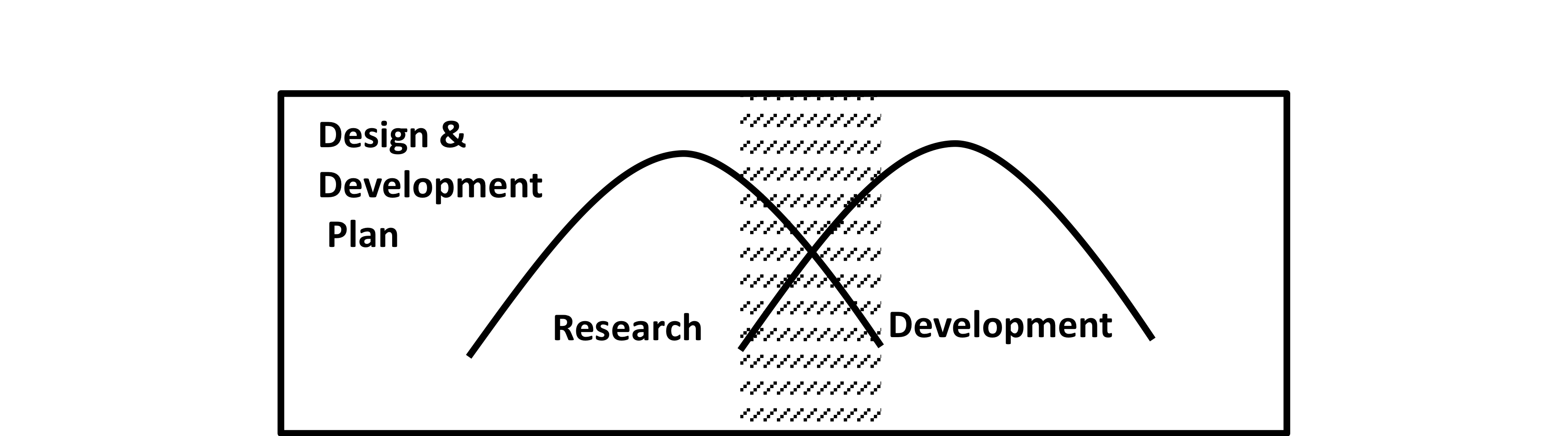
The FDA does not specify at what point in the development process when design and development planning should begin or end but the following graphical models give some clarity as to when initial planning will occur relative to research and development activities.
The two humps in Figure 5 depict the relationship and the relative amount of research and development activities performed during the design and development cycle. The shaded area is the typical time when initial development planning occurs and when the design and development plan is generated, reviewed and approved.